Manufactured by Metakem, sold by Nano Cats GmbH
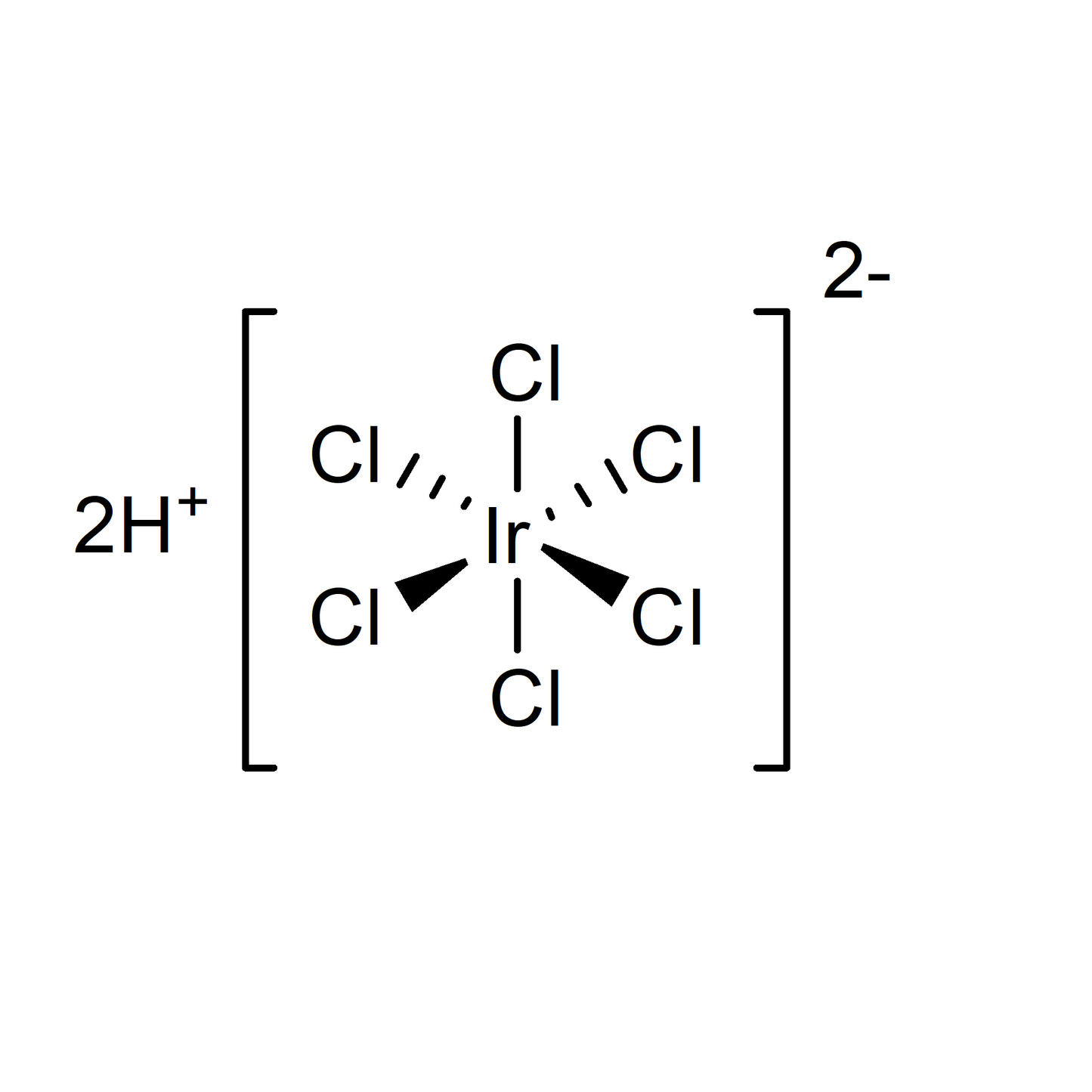
Hexachloroiridic(IV)-acid
Hexachloroiridic(IV)-acid
Couldn't load pickup availability
Hexachloroiridic acid (the anhydrous form, H₂IrCl₆) is a fundamental compound in iridium chemistry, serving as a precursor for advanced catalysts and electrode materials without the hydration component found in the hexahydrate form. Its solid form presents unique physical and chemical properties essential for high-purity industrial applications, offering enhanced stability and handling characteristics in dry environments. This anhydrous compound is particularly useful in the manufacture of corrosion-resistant coatings and in catalytic processes requiring precise stoichiometry and moisture control.
| Property / Feature | Description / Value |
|---|---|
| Chemical Formula | H₂IrCl₆ |
| Molecular Weight | 406.95 g/mol |
| Appearance | Black to dark brown crystalline solid |
| Density | Approximately 1.02 g/cm³ at 25 °C |
| Melting Point | Around 65 °C (may decompose) |
| Solubility | Soluble in water, hydrochloric acid, and alcohols |
| Stability & Storage | Stable when dry; must be kept in moisture-free environment, under inert gas recommended |
| Toxicity & Safety | Corrosive and toxic; requires protective handling |
| Key Applications | - Precursor in production of iridium-based catalysts and coated electrodes |
| - Used to prepare stable anodes in chlor-alkali and PEM electrolysis systems | |
| - Catalyst in electrochemical reactions and organic syntheses | |
| - Intermediate for producing iridium-substituted polyoxometalates | |
| Special Features | Anhydrous nature allows precise catalytic loading and better control in dry chemical processes |
Hexachloroiridic acid anhydrous offers a balance of chemical robustness and application versatility, making it indispensable in catalysis, electrochemistry, and coatings for clean energy and industrial technologies.
Share